
The electrons are fixed in place in orbit around the nucleus of two protons and two neutrons which are represented by two clear and two translucent colored 6 mm faceted glass or crystal beads. Then in 1913 Bohr, by accident, stumbled across Balmer's numerology for the hydrogen spectrum, and in a flash came up with a workable model of the atom.These helium atom earrings are based on the Bohr model of the atom. As was common with Bohr when confronted with a puzzle, this struggle was nearly all-consuming. He was struggling to make sense of all of this. He also knew about the existence of line spectra from chemical elements a document on this topic may be found here. Neils Bohr knew about all of these facts, and in the early part of the century was collaborating with Rutherford. As they lose energy they will spiral into the nucleus and in a matter of nanoseconds will collide with it. Thus the electrons is this planetary model will be radiating energy. We know from classical electromagnetic theory that any charged body that is in a state of motion other than at rest or in uniform motion in a straight line will emit energy as electromagnetic radiation. The electrons were in orbits around the nucleus, held in their orbits by the electric force that attracts negatively charged electrons to the positively charged nucleus. Soon, people proposed a planetary model of the atom. This small massive positively charged object is called the nucleus. Thus the plum pudding model of the atom collapsed: most of the mass and the positive charge of the atom was concentrated into a very small volume. Similarly, Rutherford concluded that inside the gold foil there must be something fairly small, very massive, and positively charged. If you were doing the experiment involving the BB's and the cream cheese and occasionally had a BB scattered back towards you, you would probably conclude that there was something fairly small and very massive inside the slab. Most of the alpha particles are only slightly deflected, as expected, but occasionally one is deflected back towards the source. The above diagram shows what we would expect the result of Rutherford's experiment to be if the "plum pudding" model of the atom is correct. However, once in a while he observed an alpha particle that was scattered right back towards the radioactive source. And in fact the results of Rutherford's experiments usually followed this model: almost all of the alpha particles emerged on the other side slightly deflected by their interaction with the gold. If Thomson's plum pudding model was correct, the experiments would be sort of similar to firing BB's from a BB gun into a thin slab of cream cheese with chives. He was directing a beam of these alpha particles onto a very thin piece of gold foil. He knew that these particles had a mass much larger than the electron and had a net positive electric charge now we know that these particles are identical to the nucleus of the helium atom.
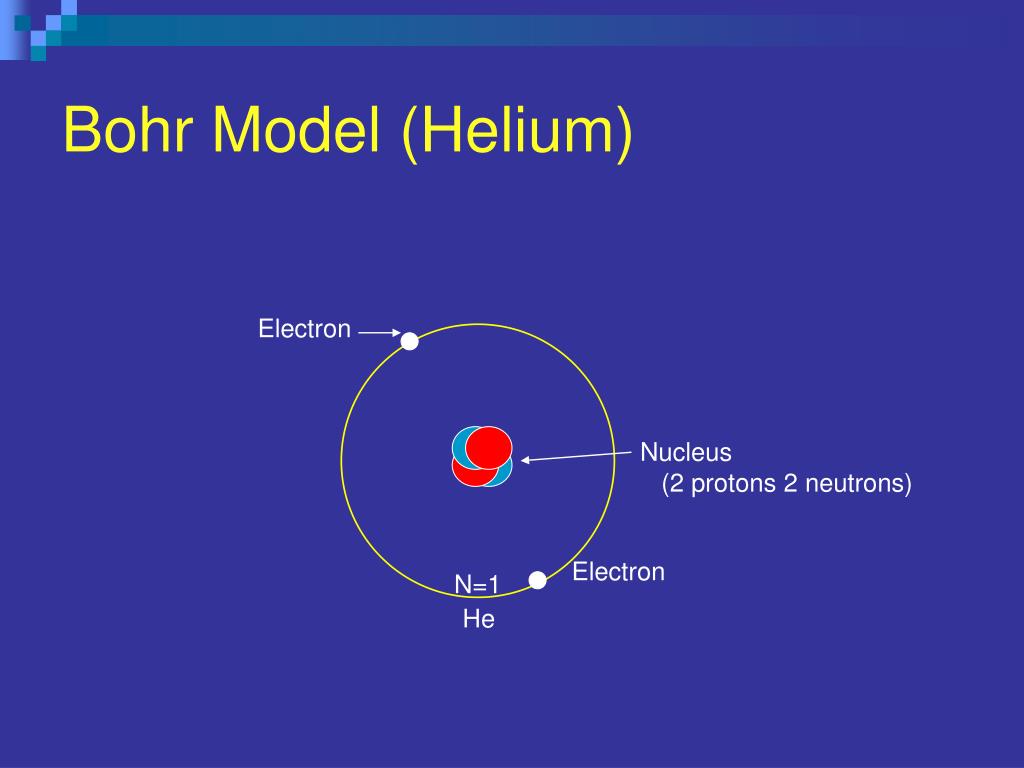
In the early twentieth century Rutherford was experimenting with one of the newly discovered radioactive substances, one that emitted alpha particles. Hydrogen, for example, has one electron helium has two carbon has six, etc.

The number of electrons determines the particular chemical element. This was called the plum pudding model of the atom. The total charge of the electrons exactly balanced the positive charge of the large mass, so the total electric charge was zero. Thus he proposed that atoms consisted of a large massive positively charged body with a number of small negatively charged electrons scattered throughout it. He determined that these electrons had a negative electric charge and compared to the atom had very little mass. Throughout the nineteenth century atomism became an idea that came to dominate thought in a number of fields, including political science, sociology, psychology, biology and more.
Nonetheless opposition from critics such as Mach, who never believed in atoms, was largely ignored.

Dalton "proved" his theory with a number of assumptions, each of which is either factually wrong or was used in a logically inconsistent manner. \)īy the late nineteenth century, most people had accepted Dalton's proposal of 1808 that matter was made of atoms.


 0 kommentar(er)
0 kommentar(er)
